
Tissue preparation - Fixatives
The chemical formulas for the most common fixatives are shown in this image.
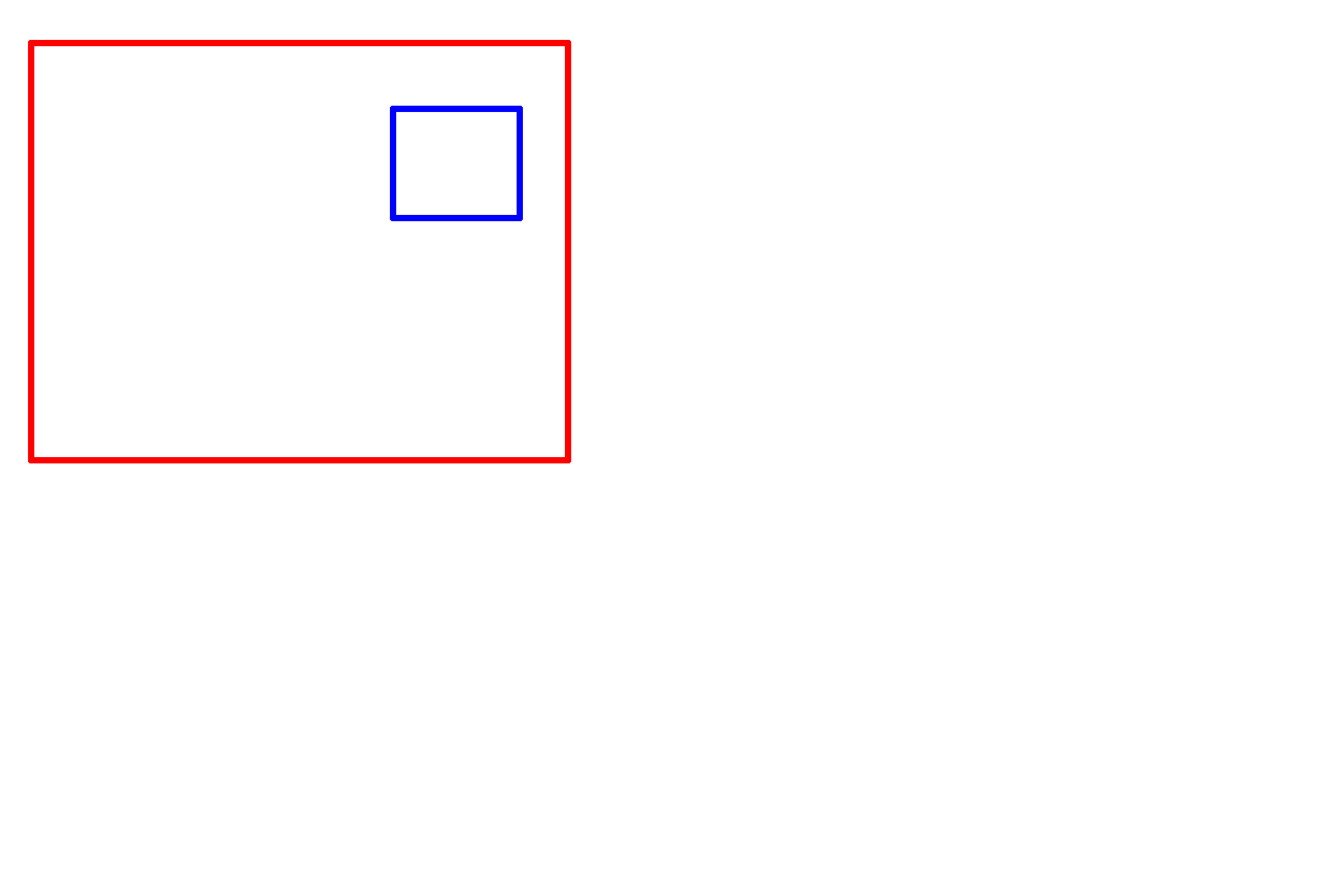
Formaldehyde >
Formaldehyde is a single carbon, mono-aldehyde that cross-links and stabilizes protein primarily by reacting with primary amino groups. Formaldehyde is used in a solution called formalin.
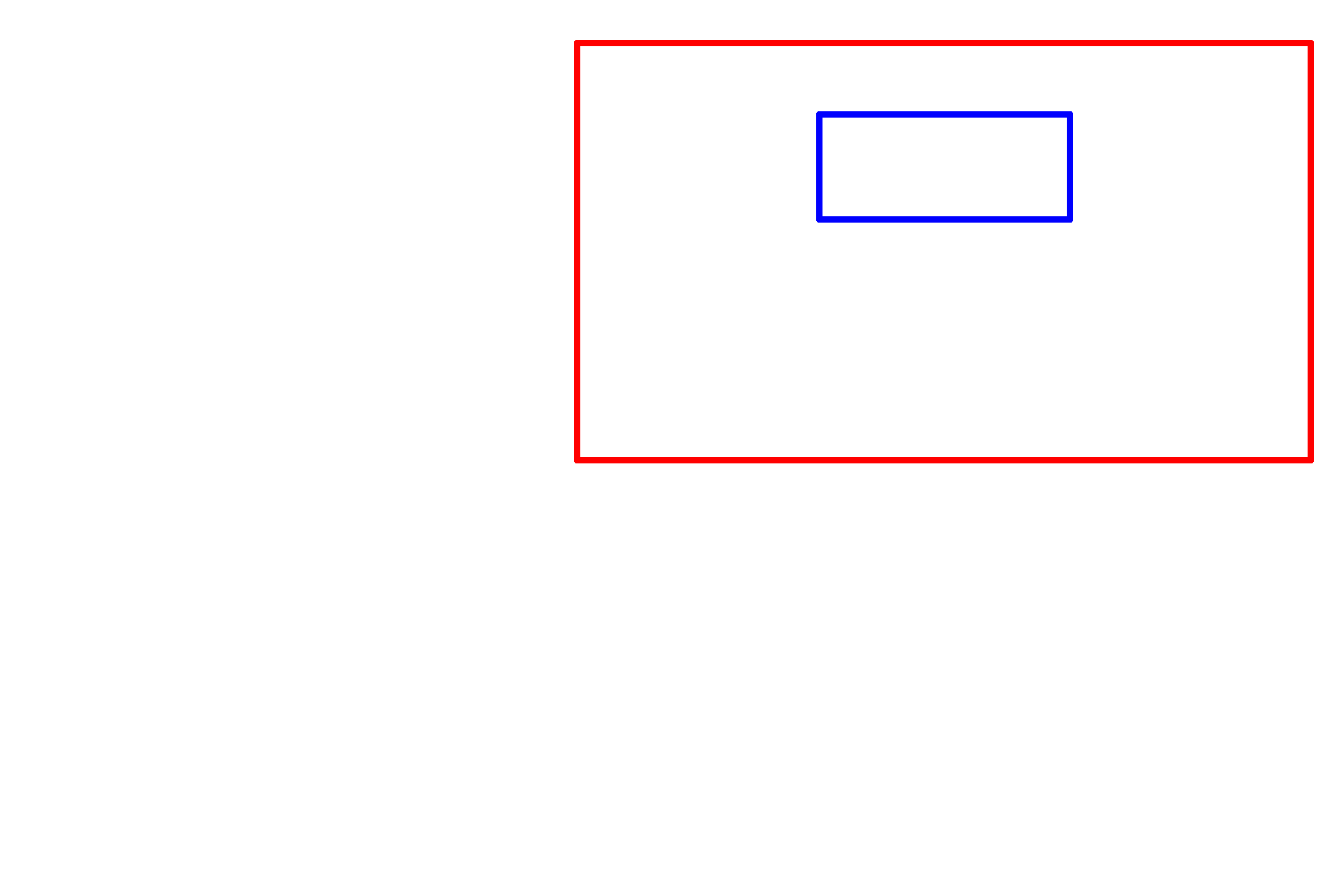
Glutaraldehyde >
Glutaraldehyde is a di-aldehyde which, like formaldehyde, cross-links and stabilizes protein. Because it is bi-functional, it provides superior fixation compared to formaldehyde. Glutaraldehyde is required for electron microscopy and is often used in combination with formaldehyde.
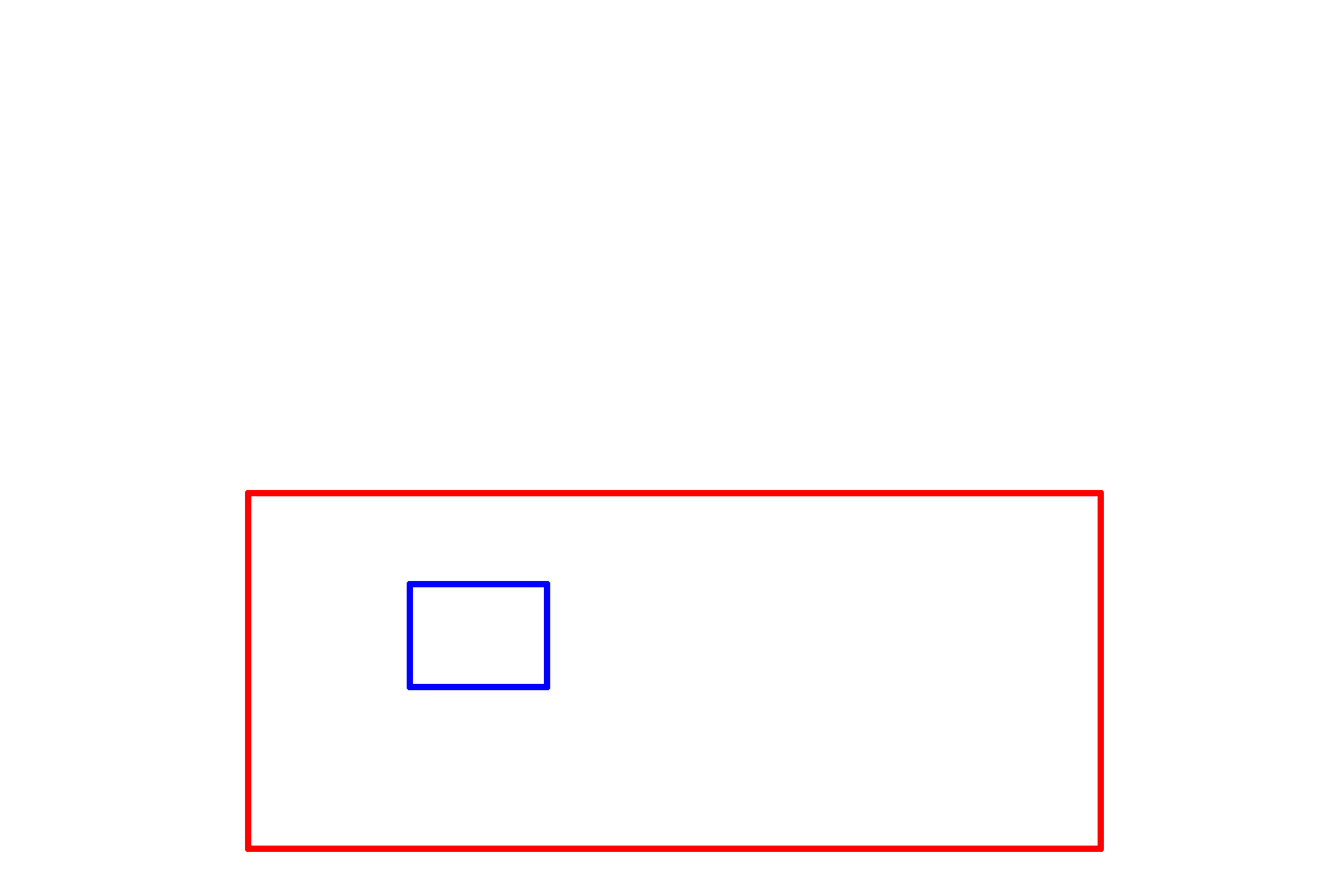
Osmium tetroxide >
Formaldehyde and glutaraldehyde are primarily protein fixatives. To stabilize and retain lipid, fixation with osmium tetroxide is used. Osmium reacts with unsaturated double bonds in fatty acids and since osmium is a heavy metal, it also adds contrast to the tissue. Osmium fixation is routinely used in electron microscopy.